Do you suffer from Urinary Incontinence?
Urinary Incontinence is the involuntary leakage of urine. 1 in 3 women over the age of 50 years old suffer from SUI.
Get started to see if you qualify!
About the Study
How Does it Work?
Step 1
Complete our sign up form to see if you qualify for our Clinical Trial.
Step 2
We will connect you with a Clinical Trial site in your area.
Step 3
Become one of thousands of patients taking part in improving the quality of life for women.
Sign Up
Participants may receive compensation or their time and travel.
What happens if you sign up? We will match you to a research center in your area that needs volunteers with Urinary Incontinence or notify you when one becomes available. The study team will then contact you, and you may have the opportunity to participate if qualified. If you think you might like to participate in the Women’s Pelvic Health Study or would like more information, please enter your information below so we can see if you may qualify and can contact you about the study. Keep in mind that participation is entirely voluntary.
What is Urinary Incontinence?
The bladder and urethra are supported by a group of pelvic muscles and connective tissue. As these muscles weaken, the bladder and urethra lose their support and move out of position, allowing urine to leak. It is a misconception that this is a normal part of aging. In fact, it can happen at any age and, while common, is not normal.
Types of Urinary Incontinence
Sign Up Today
Frequently Asked Questions
Will future pregnancies affect the results of my sling?
Future pregnancies may negate the outcome of your sling procedure. Another procedure may be needed to regain continence.
Will I need to stay in the hospital after the procedure?
A hospital stay is usually not required for this procedure; however, depending on your condition, your physician may require you to stay overnight.
If you are having another procedure performed at the same time, you may be required to stay longer. Ask your physician about what to expect.
How long does the sling procedure last?
The mesh material is permanent; however, the durability of mesh repair for urinary incontinence depends on many factors. Most clinical studies have collected outcomes data for only 1-2 years; these studies suggest that the majority of women who undergo mesh repair for incontinence report their symptoms as less bothersome compared to before their surgery. The mid urethural sling has been studied as long in follow-up as any other procedure for SUI and has demonstrated superior safety and efficacy. This includes a recent 7 year follow-up study.
When will I see the results?
In most cases, you will see results immediately after the procedure with little pain or discomfort. Many women are able to return to their daily activities within several days.
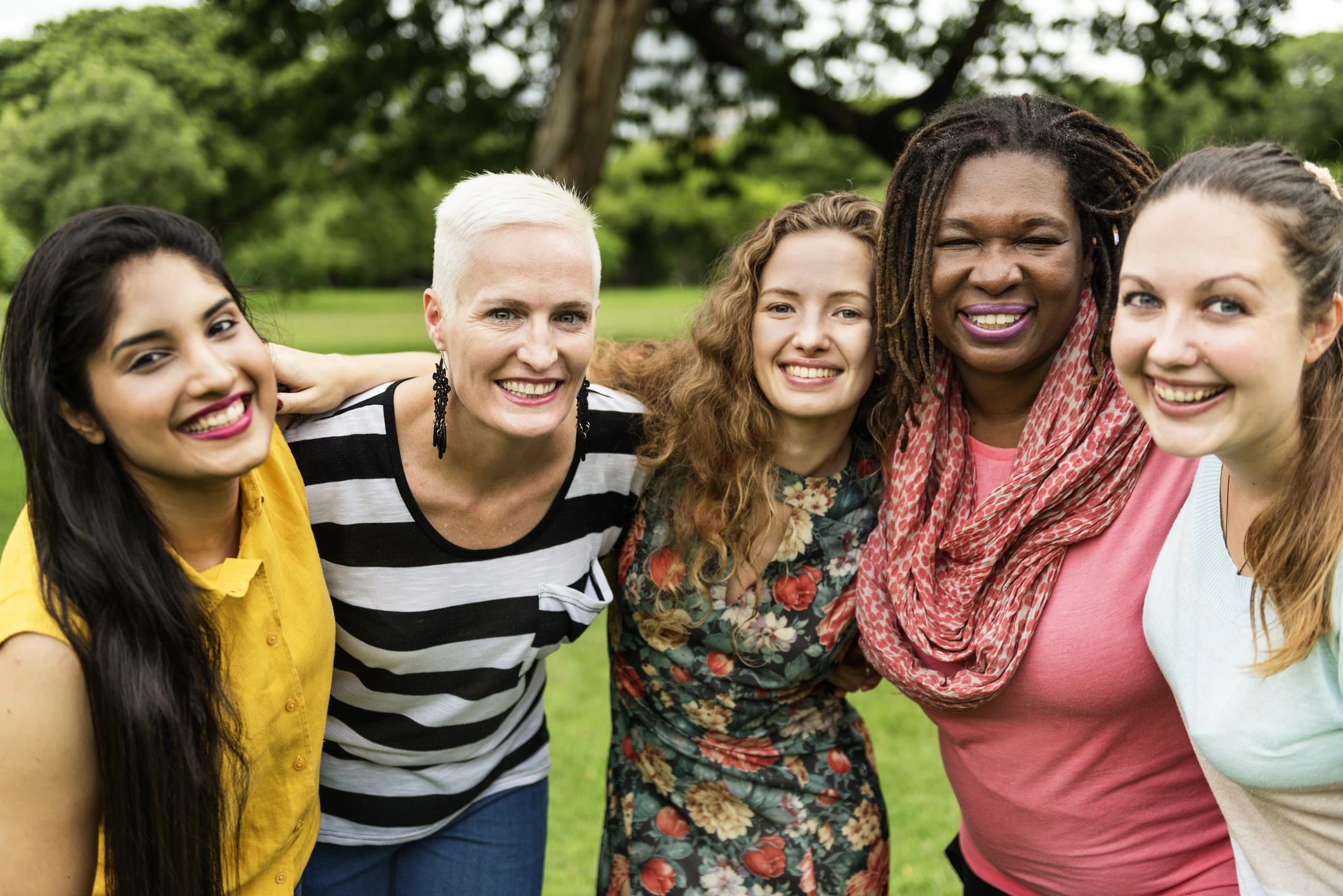
Indication
Desara is intended to be used in females to position a mesh for treatment of Stress Urinary Incontinence (SUI), mixed incontinence resulting from urethral hypermobility or intrinsic sphincter deficiency, and vaginal vault prolapse.
Adverse reactions
Potential adverse reactions are similar to those associated with other surgically implanted meshes. Adverse reactions include but are not limited to potential for infection, inflammation, adhesions, fistula formation and extrusion. Perforations or lacerations of vessels, nerves, bladder, bowel, and uterus may occur during passage of any needles and may require open surgical repair.
Contraindications
- As with any suspension procedure, this device must not be implanted in patients while on anticoagulants, aspirin, non-steroidal anti-inflammatory agents, or in those with bleeding disorders.
- This mesh will not stretch significantly; therefore, it must not be utilized in patients with future growth potential, including women with plans for future pregnancies.
- If this device is used in contaminated wounds subsequent infection may require removal of mesh.
Warnings
- Meshes are provided by CALDERA MEDICAL as a sterile product. They’re intended for single use only. Do not re-sterilize. Discard any unused product. If packaging is opened or damaged it is no longer considered sterile and should not be used clinically.
- Polypropylene should not be placed in contact with bowel or visceral organs including the urinary bladder.
- Before utilizing this product, the surgeon must be familiar with the surgical techniques for bladder neck suspensions and vaginal vault prolapse. Please review surgical technique for further details before use.
- Retropubic bleeding may occur postoperatively as with any needle suspension procedure. Observe for any symptoms or signs before the patient is released from the hospital.
- Cystoscopy is mandatory and must be performed to confirm bladder integrity and to recognize any inadvertent bladder perforation.
- As with all sling procedures, the patient should be counseled that future pregnancies may negate the effects of the surgical procedure and the patient may again become incontinent.
Learn More
Desara ® One Single Incision Sling 522 Study – Full Text View – ClinicalTrials.gov
Leak Free Study - 2025 - All rights reserved